Global Lung Cancer Liquid Biopsy Market Size And Overview
According to the latest market study by the CMI Team, the Global Lung Cancer Liquid Biopsy Market is anticipated to achieve a CAGR of 10.2% during the forecast period from 2024 to 2033. The market size in 2024 is estimated to be valued at USD 504.8 Million. By 2033, the market size is expected to reach USD 1,210 Million.
Global Lung cancer liquid biopsy market is the industry within the healthcare sector that specializes in the research, production, and sale of diagnostic tests that examine biological fluids (e.g., blood or saliva) for biomarkers related to lung cancer.
The tests provide a non-invasive alternative to conventional tissue biopsies, allowing for early detection, disease monitoring, and treatment decision-making guidance.
The market has multiple players such as diagnostic companies, pharmaceutical companies, research centers, and healthcare providers that help develop and make liquid biopsy technology more accessible to manage lung cancer patients better.
Growth Drivers for Global Lung Cancer Liquid Biopsy Market
The Lung Cancer Liquid Biopsy Market has immense opportunities for growth on account of numerous factors:
Advancements in Technology Continuing developments in liquid biopsy platforms, including advancements in highly sensitive and specific assays, propel growth in the market through enhanced efficiency and accuracy in the detection and monitoring of lung cancer.
Shift to Personalized Medicine: Growing uptake of personalized medicine strategies, which depend on molecular characterization of tumors to guide treatment decisions, drives the need for liquid biopsy tests that yield actionable genomic data specific to each patient.
Increasing Prevalence of Lung Cancer: The increasing worldwide burden of lung cancer, especially non-small cell lung cancer (NSCLC), acts as a strong growth driver for the liquid biopsy market, where early detection and monitoring of the disease become all the more essential for better patient outcomes.
Lung Cancer Liquid Biopsy Market Major Threats
The Lung Cancer Liquid Biopsy Market is exposed to some of the following serious threats that would affect its growth and profitability in the coming times. These include:
Technological Limitations: In spite of the developments, existing liquid biopsy technologies could possess some sensitivity and specificity limitations, which might generate false-positive or false-negative test results. This might jeopardize faith in the dependability of liquid biopsy testing and its subsequent adoption in the clinic.
Regulatory Hurdles: Tough regulatory norms for the clearance and validation of liquid biopsy tests are a major market entry obstacle for new entrants. Delays or failures in getting regulatory clearances may slow down innovation and restrict the supply of validated liquid biopsy assays for lung cancer diagnosis and monitoring.
Competition from Conventional Tissue Biopsies: Conventional tissue biopsies are still the gold standard for lung cancer diagnosis and can remain the preferred choice for some physicians based on familiarity and perceived dependability. Competition from tissue biopsies may hinder the uptake of liquid biopsy testing, particularly in areas where tissue biopsy centers are easily accessible.
Category-Wise Insights:
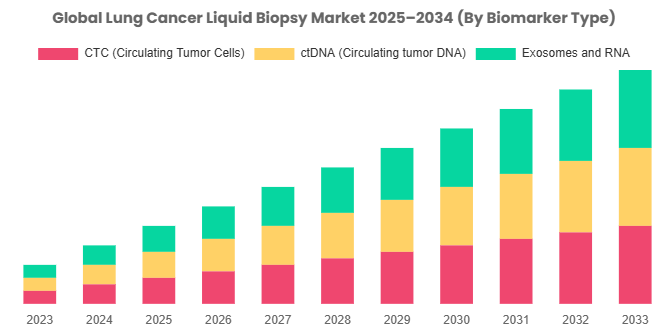
By Biomarker Type
CTC (Circulating Tumor Cells): Circulating Tumor Cells (CTCs) are tumor cells that have broken away from the original tumor and entered the bloodstream. In the Lung Cancer Liquid Biopsy Market, there is a growing interest in isolating and examining CTCs because of their potential to shed rich information about disease development, metastasis, and therapeutic response. This trend indicates the advancement of novel technologies for CTC capture, counting, and molecular profiling, thus enabling personalized therapeutic approaches and enhancing outcomes for patients.
ctDNA (Circulating tumor DNA): Circulating tumor DNA (ctDNA) is fragmented DNA from tumor cells released into the blood. In the Lung Cancer Liquid Biopsy Market, ctDNA testing has come as a potential non-invasive tool for the identification of genetic mutations and genomic changes related to lung cancer. The trend is driving the uptake of liquid biopsy as a go-to method for the early diagnosis, treatment choice, and tracking of treatment response among lung cancer patients. The growth of this segment is further fueled by the development of sensitive and specific ctDNA detection assays and the discovery of new biomarkers.
Lung Cancer Liquid Biopsy Market Regional Analysis
The Lung Cancer Liquid Biopsy Market is divided into several regions, such as North America, Europe, Asia-Pacific, and LAMEA. Below is a brief summary of each region:
North America: In North America, comprising the United States and Canada, key trends in the lung cancer liquid biopsy market involve a high penetration of sophisticated medical technologies. The region experiences substantial focus on the personalized medicine domain, fueling the need for liquid biopsy tests customized to unique patient profiles. Moreover, the region has good regulatory systems as well as reimbursement policies favoring the adoption of liquid biopsy within the normal practice of medicine.
Europe: European trends in the Global Lung Cancer Liquid Biopsy Market for lung cancer are characterized by the rising incidence of lung cancer and the expanding awareness of non-invasive diagnostic testing. Healthcare innovation is a priority in Europe, which has spurred investments in the R&D of liquid biopsy technologies. Academic, industrial, and healthcare collaboration promotes validation and the uptake of liquid biopsy assays throughout Europe.
Asia-Pacific: In the Asia-Pacific region, such as China, Japan, and India, the trends in the global Lung cancer liquid biopsy market are influenced by fast urbanization, aging populations, and increasing healthcare spending. There is an increasing need for affordable and non-invasive diagnostic tests, which is driving the use of liquid biopsy testing. Government efforts to enhance cancer screening and treatment access also drive market growth in the region.
LAMEA (Latin America, Middle East, and Africa): Trends in the global Lung cancer liquid biopsy market in the LAMEA region are driven by multicultural socioeconomic conditions and healthcare infrastructure gaps. Access to sophisticated diagnostics in some regions is restricted, yet there’s greater appreciation for the utility of liquid biopsy in circumventing lung cancer diagnosis and monitoring barriers. Joint initiatives by public health authorities, private sector entities, and international organizations fuel awareness and uptake of liquid biopsy technology.